 Published 6 June 2023
Published 6 June 2023
The Generic PROM Sub-committee was formed early this year, to make recommendations to the PRM Board around Generic PROM(s) selection for implementation within South Australia.
The committee is made up of a diverse group of experts from the Research Collaborative, with relevant skills, knowledge, and experience in patient-reported outcomes, both from South Australia and interstate. This includes clinicians, consumers, health economists, researchers, representatives providing interjurisdictional experiences from the Agency of Clinical Innovation (PRM Program) in New South Wales and our digital solution vendor, The Clinician.
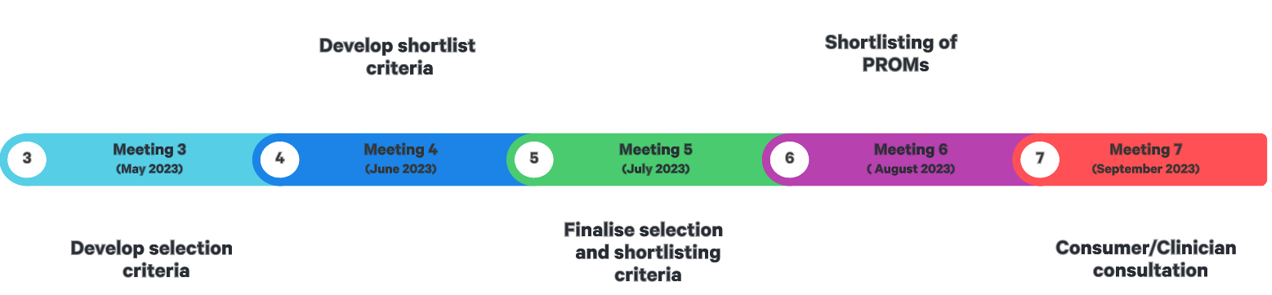
The committee will operate for approximately 6 months and has already convened twice thus far. In the past few months, the committee has made significant progress, including:
- Recruiting consumers to the committee
- Co-designing consumer involvement
- Identifying PROM instruments currently in use across healthcare settings in SA, nationally, and internationally
- Determining selection priorities and associated evaluation criteria
The group aims to meet each month but will remain connected outside of meetings.
The committee is currently working on developing selection criteria. Once this is complete, they will shortlist PROMs that meet the criteria. The committee will then consult with consumers to get their input on the shortlisted PROMs. Finally, the committee will draft recommendations for the PRM Board.
We are excited to have a diverse group guiding the recommendation of a preferred instrument for application in South Australia’s PRM Program and look forward to sharing further the outcome arising from this work.